Phage lysin compound HY-133: Start of clinical phase I “first-in-human” study to eliminate Staphylococcus aureus in the nasal microbiome
Digitally coloured scanning electron micrograph of multiple mustard-coloured, spherical Staphylococcus aureus bacteria attacked by blue-coloured human white blood cells.
The novel active agent HY-133, which was developed by HYpharm and prepared for clinical trials in collaboration with the German Center for Infection Research (DZIF) and universities in Tübingen, Munich, Münster and Greifswald, received approval in March 2024 to test its safety, tolerability and efficacy in a Phase I clinical trial. HY-133 acts specifically against Staphylococcus aureus, a bacterium that occurs in the nasal microbiome of around one in three people. Colonisation is harmless in normal life, but can become a problem during operations and hospital stays. The active agent is designed to combat colonisation of the nose with S. aureus, including methicillin-resistant S. aureus (MRSA) strains, and thus the risk of its clinical spread both in patients and in the hospital environment. The “first-in-human” study with the novel drug candidate began on 10 July with the recruitment of clinically healthy volunteers who tested positive for S. aureus colonisation of the nose.
Bacteriophages—phages for short—are viruses that can specifically kill bacteria. They are a potentially valuable addition to antibiotic therapy in the targeted treatment of infectious diseases. The novel active agent HY-133 is a phage lysin—a protein from a phage that attacks and lyses Staphylococcus aureus bacteria, including methicillin-resistant S. aureus (MRSA) strains, in a highly specific manner. The lysing protein was produced artificially and optimised as a “designer protein” under the name HY-133.
“Around 30-40% of people are naturally colonised with a Staphylococcus aureus strain in the nasal cavity,” explains Prof. Karsten Becker, microbiological specialist at Greifswald University Medicine and one of the study’s principal investigators.
“If an infection with this pathogen—for example sepsis or wound infection—occurs during hospitalisation, a very high percentage of cases are caused by precisely this strain from the nasal cavity. If it is an MRSA strain, antibiotic therapy is made even more difficult by its multi-resistance. In addition, a high level of hospital hygiene is required to prevent the spread of an MRSA strain in the hospital. In order to minimise these risks, an effective means of removing the pathogen from the nasal cavity is required. However, the nasal ointment that has primarily been used to date only achieves pathogen elimination after multiple applications over several days and, as an antibiotic-containing agent, it is itself subject to resistance development,” continues Prof Becker.
Testing of the new active agent has been driven forward since 2010 by DZIF scientists Prof. Becker at Greifswald University Medicine (formerly at Münster University Hospital) and microbiologist and DZIF executive board member Prof. Andreas Peschel at the University of Tübingen in cooperation with Hyglos (HYpharm's predecessor organisation)—since 2012 as part of the DZIF research area Antibiotic-resistant and Healthcare-associated Bacterial Infections. Due to the successful preclinical development of the active agent, authorisation for a first clinical trial in humans was granted by the German Federal Institute for Drugs and Medical Devices in March 2024.
“We were able to prove that HY-133 is able to destroy Staphylococcus aureus cells within a very short time, i.e. in just a few minutes, regardless of whether it is an MRSA strain or not,” emphasises Prof. Becker and continues: “It is particularly advantageous that the phage lysin does not affect other microorganisms and therefore does not affect the so-called ‘normal flora’—we now call it microbiota—in the nasal cavity.” “We also want to substantiate this with the clinical study,” adds Prof. Peschel.
The substance was prepared for clinical testing by DZIF scientists Prof. Evgeny A. Idelevich and Prof. Becker from the Friedrich Löffler-Institute for Medical Microbiology in Greifswald and Prof. Peschel in Tübingen. The DZIF provided more than five million euros for the GMP production, preclinical testing and development of a stable formulation at the Ludwig-Maximilians-Universität München (Prof Dr Gerhard Winter) through to the implementation of clinical phase I.
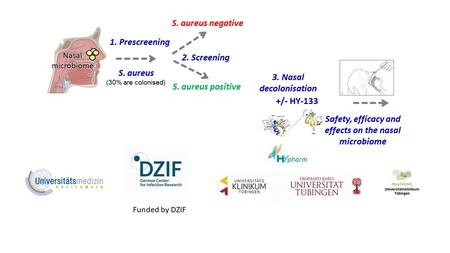
Under the responsibility of Dr Sebastian Volc, the Phase I clinical trial began on 10 July 2024 at the Study Centre Immunodermatology at the University Hospital of Tübingen (sponsor of the study). The randomised, double-blind, placebo-controlled “first-in-human” study with single and multiple doses is investigating the safety, tolerability and efficacy of HY-133. In addition, the effect of HY-133 on the natural microbiome of the nose is being tested. The study has now started with the recruitment of the first test subjects—clinically healthy volunteers who have tested positive for nasal colonisation with S. aureus but do not show any symptoms of disease.
Further information can be found in the DZIF press release from 11 June 2024.